A) decreased hydrogen ions can break chemical bonds.
B) decreased hydrogen ions can change the shape of large complex molecules, rendering them nonfunctional.
C) decreased hydrogen ions can disrupt tissue functions.
D) decreased hydrogen ions can kill living cells.
E) all of the above
G) B) and E)
Correct Answer

verified
E
Correct Answer
verified
Multiple Choice
Covalent bonds are formed when
A) atoms share electrons.
B) cations and anions are held together by their opposite charges.
C) a pair of electrons is shared unequally by two atoms.
D) hydrogen forms bonds with negatively charged atoms in the same or different molecules.
E) two or more atoms lose electrons at the same time.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement about the reaction H2 + Cl2 → 2HCl is correct?
A) H2 and Cl2 are the products.
B) HCl is the product.
C) One molecule of hydrogen contains one atom.
D) One molecule of chlorine contains one atom.
E) All of the above are correct.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a characteristic of hydrogen bonds?
A) Hydrogen bonds are strong attractive forces between hydrogen atoms and negatively charged atoms.
B) Hydrogen bonds occur ONLY in water.
C) Hydrogen bonds can form between neighboring molecules.
D) Hydrogen bonds are part of fatty-acid structure.
E) Hydrogen bonds are part of carbohydrate structure.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is unique to RNA?
A) glucose
B) phosphate group
C) ribose
D) adenosine triphosphate
E) deoxyribose
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Short Answer
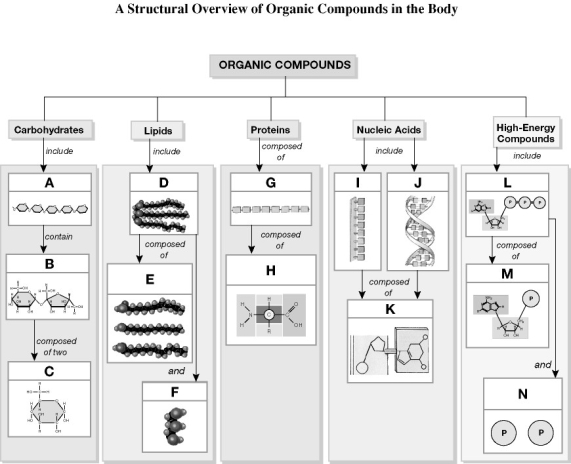 Using the figure above, identify the labeled part.
-Label L: ________
Using the figure above, identify the labeled part.
-Label L: ________
Correct Answer

verified
Correct Answer
verified
Short Answer
_________________________ are soluble inorganic compounds whose ions will conduct an electric current in solutions.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a weak electrical attraction between molecules?
A) ionic bond
B) covalent bond
C) polar bond
D) metallic bond
E) hydrogen bond
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A solution containing more hydrogen ions than hydroxide ions is
A) acidic.
B) basic.
C) neutral.
D) alkaline.
E) none of the above
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The most important metabolic fuel molecule in the body is
A) sucrose.
B) starch.
C) protein.
D) vitamin B12.
E) glucose.
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The best definition of organic material is anything that
A) contains carbon.
B) contains carbon, oxygen, and hydrogen covalently bonded.
C) contains carbon, nitrogen, and hydrogen covalently bonded.
D) contains hydrogen covalently bonded.
E) contains carbon and hydrogen covalently bonded.
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Short Answer
Chemical reactions that occur in the human body are controlled by special protein molecules called _________________________.
Correct Answer

verified
enzymes
Correct Answer
verified
Multiple Choice
AB → A + B is to decomposition as A + B ↔ AB is to
A) exchange.
B) reversible.
C) combustion.
D) replacement.
E) metabolism.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
All of the elements and compounds that are eaten and used by the body for some function are called
A) inorganic compounds.
B) organic compounds.
C) nutrients.
D) metabolites.
E) enzymes.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A class of lipids used to signal cells to undergo changes is called
A) steroids.
B) phospholipids.
C) triglycerides.
D) hormones.
E) monoglycerides.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following would have a negative charge?
A) an atom
B) a molecule
C) a proton
D) a neutron
E) an electron
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Short Answer
 Using the figure above, identify the labeled part.
-Label J: ________
Using the figure above, identify the labeled part.
-Label J: ________
Correct Answer

verified
Correct Answer
verified
Short Answer
 Using the figure above, identify the labeled part.
-Label H: ________
Using the figure above, identify the labeled part.
-Label H: ________
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many electrons do MOST atoms need in their outer shell to be stable?
A) two
B) three
C) four
D) six
E) eight
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If an element is composed of atoms with an atomic number of 8 and a mass number of 14, then a neutral atom of this element contains
A) 6 protons.
B) 6 neutrons.
C) 6 electrons.
D) 14 protons.
E) 14 electrons.
G) A) and C)
Correct Answer

verified
B
Correct Answer
verified
Showing 1 - 20 of 106
Related Exams