A) 328.0 days
B) 410.0 days
C) 82.00 days
D) 164.0 days
E) none of these
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When the nucleus of an element undergoes beta emission
A) the mass number increases by one.
B) the mass number decreases by one.
C) the atomic number decreases by one.
D) the number of neutrons increases by one.
E) the atomic number increases by one.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A scintillation counter measures radiation by detecting
A) cations produced from radiation colliding with phosphor gases.
B) electrons released when gas atoms are ionized by the radiation.
C) alpha and beta particles as they strike a detector window.
D) the increase in temperature when a gas is struck by radiation.
E) flashes of light emitted from a phosphor affected by radiation.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which particle does the nuclide symbol 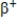 represent?
represent?
A) positron
B) helium nucleus
C) electron
D) proton
E) gamma photon
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Strontium-90 is produced in nuclear explosions.It can replace calcium in the bone.The half-life of 90Sr is 27.7 years.If the activity of 90Sr in the bones of an exposed person were 90 disintegrations per second,how long would it take the activity of 90Sr to decrease to 8.1 disintegrations per second?
A) 96 years
B) 66 years
C) 49 years
D) 59 years
E) 75 years
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For which of the following radioactive decay processes does the atomic number not change?
A) electron capture
B) positron emission
C) alpha emission
D) gamma emission
E) beta emission
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following radioactive isotopes is not commonly used in medical applications?
A) technetium-99m
B) iodine-131
C) strontium-90
D) cobalt-60
E) thallium-201
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is the most likely mode of radioactive decay for the radioactive nuclide carbon-14?
A) gamma emission
B) alpha emission
C) electron capture
D) positron emission
E) beta emission
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The radioactive nuclide,22Na,undergoes decay by emitting a positron.What is the nuclear composition of the product nuclide?
A) 12 protons and 10 neutrons
B) 11 protons and 11 neutrons
C) 11 protons and 10 neutrons
D) 9 protons and 12 neutrons
E) 10 protons and 12 neutrons
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
55Mn can be prepared by electron capture from which of the following?
A) (56Mn)
B) (55Fe)
C) (55Cr)
D) (51V)
E) (57Co)
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Showing 81 - 90 of 90
Related Exams