A) +1 only
B) -1, 0, and +1
C) 0 and +1 only
D) -1 and +1 only
E) 0 only
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Interhalogen compounds ________.
A) are exceedingly reactive
B) contain halogens in both positive and negative oxidation states
C) are powerful oxidizing agents
D) all of the above
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
What group 5A element is the least metallic?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Tetraboric acid,H2B4O7,is prepared by heating boric acid,H3BO3 (a condensation reaction involving water loss) .If 400.0 mmol H3BO3 are used,what mass (g) of H2O is formed,assuming quantitative stoichiometric conversion?
A) 5.77
B) 0.500
C) 0.320
D) 7.21
E) 9.01
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which form of elemental sulfur is the most stable at room temperature?
A) rhombic sulfur
B) monoclinic
C) hexagonal
D) triclinic
E) tetraclinic
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following xenon compounds: (i) XeCl2 (ii) XeCl4 (iii) XeO4 (iv) XeOCl4 (v) XeO3 Which of the compounds is(are) polar?
A) (i) only
B) (iii) and (iv)
C) (iv) and (v)
D) (ii) and (iii)
E) (iv) only
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The prefix thio- denotes ________.
A) replacement of an oxygen atom by a sulfur atom
B) a sulfur-sulfur double bond
C) sulfur in a negative oxidation state
D) a sulfur-oxygen double bond
E) an allotropic form of sulfur
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which noble gas is known to form a variety of binary compounds?
A) Xe
B) He
C) Ne
D) Ar
E) Kr
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The oxidation numbers of sulfur in the sulfate ion,sulfite ion,sulfur trioxide,and hydrogen sulfide are ________,________,________,and ________,respectively.
A) +4, -2, +4, +6
B) +6, +2, +4, +6
C) +6, +4, +6, -2
D) +4, +6, +4, -2
E) -2, +6, -2, 0
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The principal combustion products of compounds containing carbon and hydrogen in the presence of excess O2 are ________.
A) CO2 and H2O
B) CO2 and H2O2
C) CO2 and H
D) C(graphite) and H2
E) CO2 and H2
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the coefficient of NO2 when the following disproportionation reaction is balanced?
NO2 (g) + H2O (l) → 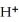 (aq) + NO3- (aq) + NO (g)
(aq) + NO3- (aq) + NO (g)
A) 1
B) 2
C) 3
D) 5
E) 4
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Hydrogen can form hydride ions.Elements in group ________ typically form ions with the same charge as the hydride ion.
A) 1A
B) 2A
C) 6A
D) 7A
E) 3A
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
B2O3 is the anhydride of ________.
A) borous acid
B) diborane
C) tetraboric acid
D) boric acid
E) borax
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the formula of borax?
A) H3BO3
B) H2B4O7
C) P5O8
D) B2O3
E) Na2B4O7 ∙ 10H2O
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Most mined phosphate rock is ________.
A) used as a strong acid
B) used as a reducing agent
C) used as a detergent
D) converted to fertilizer
E) discarded as a by-product
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Soda-lime glass contains ________.
A) SiO2 and aluminum
B) SiO2, CaO, and Na2O
C) SiO2, CO2, and citric acid
D) SiO2, CO2, Na2O
E) pure SiO2
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the oxidation state of xenon in XeO2F2?
A) 0
B) +4
C) +8
D) +2
E) +6
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Essay
What is an acetylide?
Correct Answer

verified
a carbide ...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
The oxidation number of N in NO3- is ________.
A) +1
B) +2
C) +3
D) +4
E) +5
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Silicones are ________.
A) chains of alternating silicon and oxygen atoms with attached organic groups
B) three-dimensional covalent networks of SiO4 tetrahedra
C) three-dimensional covalent networks of silicon atoms
D) flat sheets of silicon atoms
E) flat sheets of silicon and hydrogen atoms
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 194
Related Exams