A) linear
B) trigonal planar
C) tetrahedral
D) trigonal bipyramidal
E) octahedral
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Draw the Lewis structure for BrCl3.What is the hybridization on the Br atom?
A) sp3d2
B) sp3d
C) sp3
D) sp2
E) sp
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the electron geometry for the molecule BrF3.
A) Linear
B) Trigonal planar
C) Tetrahedral
D) Trigonal bipyramidal
E) Octahedral
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the electron geometry (eg) and molecular geometry (mg) of PF5.
A) eg = trigonal bipyramidal,mg = trigonal bipyramidal
B) eg = octahedral,mg = octahedral
C) eg = trigonal bipyramidal,mg = tetrahedral
D) eg = tetrahedral,mg = trigonal pyramidal
E) eg = trigonal planar,mg = octahedral
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the approximate bond angle for a molecule with tetrahedral electron geometry and bent molecular geometry.
A) 180°
B) <180°
C) <109.5°
D) 109.5°
E) <120°
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Draw the Lewis structure for SO3.What is the hybridization on the S atom?
A) sp
B) sp3
C) sp2
D) sp3d
E) sp3d2
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Draw the Lewis structure for BrO4-.What is the hybridization on the Br atom?
A) sp
B) sp3d2
C) sp3d
D) sp3
E) sp2
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the molecular orbital diagram shown to determine which of the following is most stable. 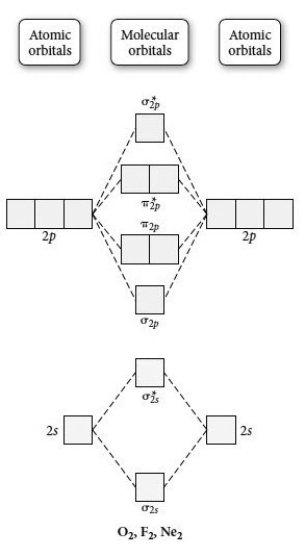
A) F2
B) F22+
C) Ne22+
D) O22+
E) F22-
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Draw the Lewis structure for BrF5.What is the hybridization on the Br atom?
A) sp3d2
B) sp3d
C) sp3
D) sp2
E) sp
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the approximate bond angle for a molecule with a tetrahedral shape.
A) 109.5°
B) 180°
C) 120°
D) 105°
E) 90°
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular geometry of NCl3?
A) T-shaped
B) tetrahedral
C) trigonal planar
D) trigonal pyramidal
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular geometry of SF5-?
A) octahedral
B) seesaw
C) square pyramidal
D) trigonal bipyramidal
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the electron geometry (eg) and molecular geometry (mg) of NCl3.
A) eg = tetrahedral,mg = tetrahedral
B) eg = linear,mg = trigonal planar
C) eg = trigonal planar,mg = bent
D) eg = linear,mg = linear
E) eg = tetrahedral,mg = trigonal pyramidal
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Draw a molecular orbital diagram and use it to determine which of the following is paramagnetic.
A) B22+
B) B22-
C) N22+
D) C22-
E) B2
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many of the following molecules have sp3 hybridization on the central atom? XeCl4 CH4 SF4 C2H2
A) 0
B) 4
C) 3
D) 2
E) 1
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Using the VSEPR model,the molecular geometry of the central atom in XeF2 is ________.
A) tetrahedral
B) trigonal bipyramidal
C) trigonal planar
D) bent
E) linear
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Draw the Lewis structure for BF3.What is the hybridization on the B atom?
A) sp
B) sp3d2
C) sp3d
D) sp3
E) sp2
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the electron geometry (eg) and molecular geometry (mg) of ICl2-.
A) eg = tetrahedral,mg = bent
B) eg = tetrahedral,mg = trigonal pyramidal
C) eg = trigonal bipyramidal,mg = linear
D) eg = trigonal bipyramidal,mg = trigonal planar
E) eg = octahedral,mg = linear
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
List the number of sigma bonds and pi bonds in a single bond.
A) 1 sigma,0 pi
B) 0 sigma,1 pi
C) 1 sigma,1 pi
D) 1 sigma,2 pi
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the compound below that contains at least one polar covalent bond but is nonpolar.
A) HCN
B) CF4
C) SeBr4
D) ICl3
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Showing 101 - 120 of 158
Related Exams