A) 177 pm
B) 354 pm
C) 500 pm
D) 1000 pm
E) 800 pm
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is considered an atomic solid?
A) F2
B) CsBr
C) N2
D) Nb
E) None of these is an atomic solid.
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the type of interaction between atoms in a nonbonding atomic solid.
A) polar bonding
B) ionic bonding
C) covalent bonding
D) hydrogen bonding
E) weak dispersion forces
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Geologists estimate that ________ percent of the Earth's crust is composed of silicates.
A) 80
B) 75
C) 85
D) 90
E) 70
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A common use of polyurethane is
A) tire cord.
B) fishing line.
C) foam cups.
D) spray-on insulation.
E) films.
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify for the rhombohedral unit cell.
A) a ≠ b = c
B) a = b ≠ c
C) a ≠ b ≠ c
D) a = b = c
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An allotrope of carbon that exists in the shape of a ball containing 36 to 100 carbons is called
A) an octahedral.
B) a nanotube.
C) a diamond.
D) a fullerene.
E) graphite.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When two waves interact with the crests of one aligning with the troughs of the other is called
A) complimentary interference.
B) destructive interference.
C) opposing interference.
D) constructive interference.
E) alignment interference.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the type of interaction between atoms in a networking atomic solid.
A) polar bonding
B) ionic bonding
C) covalent bonding
D) hydrogen bonding
E) weak dispersion forces
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An allotrope of carbon that exists as interconnected rings that assume a cylindrical shape is called
A) an octahedral.
B) a nanotube.
C) a diamond.
D) a fullerene.
E) graphite.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the element with the largest band gap.
A) lead
B) silicon
C) germanium
D) carbon
E) tin
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the radius of an Al atom (in pm) if the density of aluminum is 2.71 g/cm3.Aluminum crystallizes in a face centered cubic structure with an edge length of 2 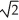 r.
r.
A) 143 pm
B) 227 pm
C) 96 pm
D) 172 pm
E) 193 pm
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the characteristics of a body-centered cubic cell.
A) edge length = 2 ![]() , 80% packing efficiency
, 80% packing efficiency
B) edge length = 2r, 52% packing efficiency
C) edge length = 4r/ ![]() , 68% packing efficiency
, 68% packing efficiency
D) edge length = 2 ![]() , 74% packing efficiency
, 74% packing efficiency
E) edge length = 2r, 60% packing efficiency
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Define blooming with chocolate.
A) mixed unevenly
B) shatters easily
C) melting unevenly
D) white residue on the surface of chocolate
E) black residue on the surface of chocolate
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the shape for SiO4.
A) tetrahedral
B) trigonal bipyramidal
C) trigonal planar
D) octahedral
E) trigonal pyramidal
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When an X-ray beam of l = 131.5 pm is incident on the surface of a copper crystal,it produces a maximum diffraction at an angle of q = 25.25 degrees.Assuming n = 1,calculate the separation between layers of copper atoms in the crystal.
A) 5.208 pm
B) 263.0 pm
C) 2.604 pm
D) 308.3 pm
E) 154.1 pm
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A common use of polyethylene is
A) tire cord.
B) fishing line.
C) foam cups.
D) spray-on insulation.
E) films.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the element with the smallest band gap.
A) lead
B) silicon
C) germanium
D) carbon
E) tin
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify an element that is NOT in nonoxide ceramics.
A) nitrogen
B) silicon
C) aluminum
D) boron
E) carbon
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the technique that determines the arrangement of atoms and measures the distance between them.
A) x-ray diffraction
B) ultraviolet
C) nuclear magnetic resonance
D) infrared
E) atomic absorption
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 81
Related Exams