A) NaBH4/H2O
B) LiAlH4/ether,then H3O+
C) PCC/CH2Cl2
D) Zn,H3O+
E) H2,Pt
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the reagents listed below would serve as the basis for a simple chemical test to distinguish between CH3CH=CHCH2OH and CH3CH2CH2CH2OH?
A) CrO3 in H2SO4
B) Cold conc.H2SO4
C) Br2 in CCl4
D) NaOH/H2O
E) NaBH4
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The final product,E,in the following reaction sequence is, 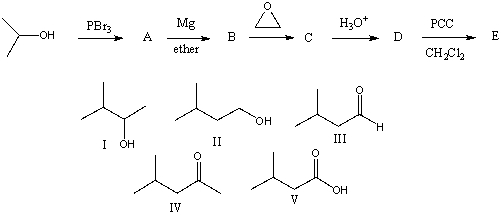
A) I
B) II
C) III
D) IV
E) V
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the reagents listed below would efficiently accomplish the transformation of ethyl-3-pentenoate into 3-penten-1-ol? 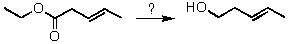
A) i) LiAlH4; ii) H2O
B) NaBH4,H2O
C) H2,Pd
D) Two of these choices.
E) All of these choices.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the reagents listed below would efficiently accomplish the transformation of CH3CH2C≡CCH2CH2CHO into CH3CH2C≡CCH2CH2CH2OH?
A) KMnO4
B) NaBH4
C) Br2 in CCl4
D) H2,Ni
E) Two of these choices.
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
List the following compounds in order of increasing level of oxidation: 
A) I,II,III
B) III,II,I
C) II,I,III
D) III,I,II
E) I,III,II
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
List the following compounds in order of increasing level of oxidation: 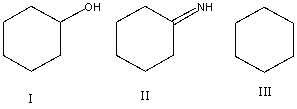
A) I,II,III
B) I,III,II
C) II,I,III
D) III,I,II
E) III,II,I
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The final product,E,in the following reaction sequence is, 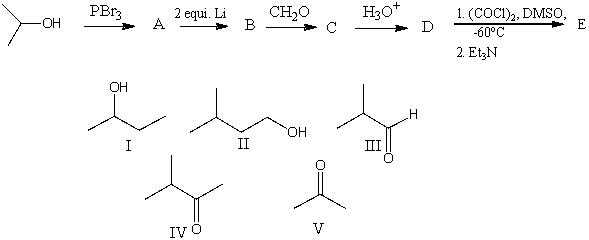
A) I
B) II
C) III
D) IV
E) V
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Short Answer
A Grignard reagent will produce a secondary alcohol when reacted with ___.
Correct Answer

verified
any aldehy...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
PCC and CrO3 are both Cr(VI)reagents.However,PCC/CH2Cl2 is useful in oxidizing primary alcohols to aldehydes,while the analogous reaction with CrO3/H2SO4 will typically produce carboxylic acids.Why?
Correct Answer

verified
Both reagents first oxidize the alcohol ...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Your task is to synthesize 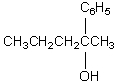 through a Grignard synthesis.Which pairs of compounds listed below would you choose as starting materials?
through a Grignard synthesis.Which pairs of compounds listed below would you choose as starting materials?
A) ![]()
B) ![]()
C) ![]()
D) More than one of these choices.
E) None of these choices.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these compounds cannot be used to prepare the corresponding Grignard reagent?
A) CH3OCH2CH2CH2Br
B) (CH3) 3CCl
C) CH2=CHCH2Br
D) (CH3) 2NCH2CH2Br
E) ![]()
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these transformations cannot be classified as a reduction?
A) RCH2Cl RCH3
B) RCH=CH2 RCH2CH3
C) RCOOH RCH2OH
D) ![]()
E) All of these are reductions.
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is (are) the principal product(s) formed when 1 mol of ethylmagnesium bromide reacts with 1 mol of 3-(N-methylamino) cyclopentanone? 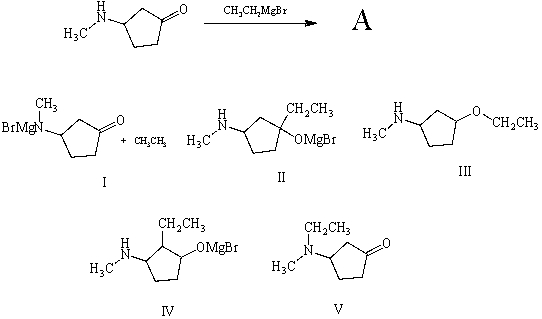
A) I
B) II
C) III
D) IV
E) V
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Essay
Suggest a reasonable synthetic strategy for the synthesis of 1,4-pentanediol from 4-hydroxybutanal HOCH2CH2CH2CHO.
Correct Answer

verified
Correct Answer
verified
Short Answer
Reaction of an alkyllithium with a ketone produces,after acid work-up,a ___ alcohol.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the reagents listed below would efficiently accomplish the transformation of CH3CH2CH=CHCH2CH2CH2OH into CH3CH2CH=CHCH2CH2CHO?
A) KMnO4,OH-
B) CrO3 / H2SO4
C) PCC in CH2Cl2
D) (i) (COCl) 2,DMSO,-60 oC,(ii) Et3N
E) Two of these choices.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following reactions would serve as a reasonable synthesis of the following racemic alcohol (2-phenyl-2-butanol) ? 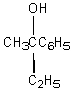
A) ![]()
B) ![]()
C) ![]()
D) Two of these choices.
E) All of these choices.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The final product,D,in the following reaction sequence, 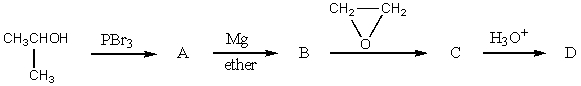 ,would be?
,would be?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
Upon reaction with a primary or secondary alcohol,the change in oxidation state of chromium ion in Jones reagent is from ___ to ___.
Correct Answer

verified
Correct Answer
verified
Showing 141 - 160 of 210
Related Exams