A) ethers.
B) carboxylic acids.
C) esters.
D) amines.
E) amides.
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which functional group is a carboxylic acid?
A) - OH
B) ![]()
C) ![]()
D) ![]()
E) - CH2 - OH
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Derivatives of which aromatic carboxylic acid have been used as analgesics, antipyretics, and anti-inflammatory agents?
A) benzoic acid
B) anthranilic acid
C) naphthenic acid
D) p-toluenesulfonic acid
E) salicylic acid
G) B) and E)
Correct Answer

verified
Correct Answer
verified
True/False
Carboxylic acids with four or fewer carbons are very water soluble.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
Sodium propionate is a common disinfectant.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name for this compound?
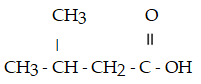
A) pentanoic acid
B) γ-methylbutanoic acid
C) 3-methylbutanoic acid
D) γ-methyl butyric acid
E) 2-methyl-4-butanoic acid
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the reaction for the ionization of β-hydroxypropanoic acid in water?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
This functional group is known as a(n) 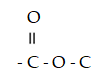
A) ester.
B) carboxylic acid.
C) alcohol.
D) aldehyde.
E) acetal.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
True/False
Benzoic acid is an aromatic carboxylic acid.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The neutralization of formic acid by NaOH produces
A) sodium formate as the only product.
B) formate ion and hydronium ion.
C) sodium formaldehyde.
D) methyl alcohol.
E) sodium formate and H2O.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The splitting apart of an ester in the presence of a strong acid and water is called
A) hydrolysis.
B) saponification.
C) neutralization.
D) esterification.
E) reduction.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
True/False
Polyesters are plastics that are used to make fabrics, bottles, and medical devices such as heart valves.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
The Krebs cycle and the citric acid cycle are different processes.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name of the following compound? 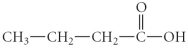
A) propyl butanoate
B) butanoic acid
C) 1-butanal
D) 1-butanoate
E) propyl methanoate
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What kind of taste do carboxylic acids have?
A) sweet
B) sour
C) fruity
D) slippery
E) oily
G) A) and E)
Correct Answer

verified
Correct Answer
verified
True/False
Carboxylic acids are strong acids.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What happens to water solubility as chain length increases in carboxylic acids?
A) It increases.
B) It decreases.
C) It stays the same.
E) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the common naming convention for carboxylic acids, what is the correct Greek letter used for the carbon adjacent to the carboxyl group?
A) α
B) β
C) γ
D) δ
E) ε
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reactants that will form an ester in the presence of an acid catalyst are
A) two carboxylic acids.
B) two alcohols.
C) a carboxylic acid and an alcohol.
D) an aldehyde and an alcohol.
E) two aldehydes.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reaction of an ester with NaOH is known as
A) esterification.
B) neutralization.
C) saponification.
D) reduction.
E) oxidation.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 83
Related Exams